Assume that only two isotopes of argon, 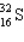 and
and 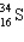 exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
Definitions:
Water Molecule
A molecule consisting of two hydrogen atoms and one oxygen atom, forming the basis of all known forms of life and many solvents.
Polar Covalent Bond
Covalent bond in which atoms do not share their electrons equally.
Young Adult
An individual in the stage of life following adolescence, typically ranging from 18 to 25 years old, focusing on personal and professional development.
Phospholipids
A type of lipid molecule that is a major component of the cell membrane, consisting of a phosphate group, a glycerol backbone, and two fatty acid tails.
Q3: Determine the percent platinum in cisplatin, PtCl<sub>2</sub>(NH<sub>3</sub>)<sub>2</sub>.<br>A)
Q11: The solubility of a gas in a
Q17: At constant T and P, in which
Q22: Name the alkane with the formula C<sub>3</sub>H<sub>8</sub>.
Q22: Calculate the time needed to plate out
Q39: Write the correct Lewis dot structure for
Q40: A 0.1 M solution of Na<sub>2</sub>SO<sub>4</sub> contains<br>A)
Q42: It is impossible to estimate the age
Q46: Ammonia is a weak base and perchloric
Q48: The electrical nature of matter can best