Consider an electrochemical cell as shown, with Zn in ZnCl2(aq) and Cu in Cu(NO3) 2(aq) , and a salt bridge containing KNO3(aq) . The overall chemical reaction is 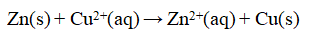
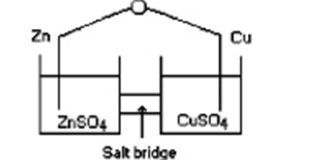
Which statement is correct?
Definitions:
Comparative Balance Sheets
Financial statements that present the financial position of an entity at different points in time, side by side, to facilitate comparison.
Q3: Which of the following is an example
Q9: All elements in the Periodic Table beyond
Q15: Which sample contains the fewest number of
Q23: Solutions form when efficient intermolecular forces can
Q27: Which represents a non-polar covalent bond?<br>A) H-O<br>B)
Q28: Which of the following are constitutional isomers?<br>A)
Q37: Arrange these elements in order of increasing
Q45: Major technical obstacle(s) to the achievement of
Q47: Alpha particles are best described as<br>A) neutral
Q51: At a given temperature, the kinetics parameters