Calculate the value of DS for the reaction shown: 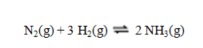 At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
Definitions:
Autonomy
The capacity of an individual to make an informed, un-coerced decision; often associated with independence and self-governance.
Extrinsic Motivation
The drive to perform or engage in an activity due to external rewards or pressures, rather than for the enjoyment or satisfaction derived from the activity itself.
Psychological Needs
Fundamental emotional or mental requirements for well-being and overall mental health, such as the needs for belonging, love, esteem, and self-actualization.
Growth And Development
The process of physical maturation and psychological and emotional changes that occur over the course of a human life.
Q19: The equilibrium constant expression for the reaction
Q20: Which substance is an oxidizing agent in
Q24: All of the following properties of a
Q30: Which of the following substances is homogeneous?<br>A)
Q31: Which of the following could not be
Q34: What is the electron configuration of Li<sup>+</sup>?<br>A)
Q44: Which of the following is not true
Q45: Give an example of an Alkaline Earth
Q46: The reaction below is an example of
Q50: Which of the following is heterogeneous?<br>A) black