Calculate the value of DS for the reaction shown: 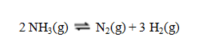 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
Definitions:
New Information
Fresh data or knowledge acquired that was previously unknown to the recipient.
Piagetian Process
A reference to the developmental and cognitive processes described by psychologist Jean Piaget, including stages of cognitive development and mechanisms of learning.
Accommodation
In cognitive development, the process by which individuals revise or adapt their cognitive schemas based on new experiences.
Psychosocial Development
A theory proposed by Erik Erikson that describes how individuals evolve through various stages of social and psychological development from infancy to adulthood.
Q1: Which of the following represents a solution?<br>A)
Q3: Which element has an atomic number of
Q7: At a particular temperature, as the pressure
Q11: Determine the ammonium ion concentration of a
Q12: In an endothermic reaction, heat is transferred
Q28: Which process is exothermic?<br>A) freezing rain drops<br>B)
Q29: Each electron in an atom or ion
Q32: Amines have the general formula<br>A) R-CHO<br>B) CH<sub>3</sub>-(CH<sub>2</sub>)<sub>n</sub>-CH<sub>3</sub><br>C)
Q37: Name the compound with the formula VCl<sub>3</sub>.
Q41: If two reactions are coupled, then<br>A) A