Calculate the value of DS for the reaction shown: 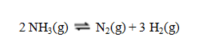 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
Definitions:
Line Graph
A type of chart used to display information as a series of data points connected by straight line segments, often used to show trends over time.
Horizontal Scale
The expansion of a company or system by adding new units or locations at the same level of operation.
Perception
The process by which individuals interpret and organize sensory information to understand their environment.
Visual
Relating to seeing or sight, often used in the context of graphical information or media.
Q6: Which statement about crystalline solids is false?<br>A)
Q11: Consider the exothermic reaction at equilibrium: <img
Q20: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q30: A decomposition reaction occurs when _ reactant(s)
Q31: The Roman numerals in the equation given
Q33: Calculate the pH of a 0.051 M
Q43: Calculate the value of E<sub>cell</sub> for
Q54: How many moles of oxygen will be
Q56: Which element is most reactive?<br>A) Cu<br>B) Fe<br>C)
Q56: If 935 g of cesium reacts with