Recovering aluminum directly from its ore, which is primarily aluminum oxide, involves the following reaction, for which thermodynamic data is tabulated below: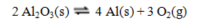

a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
Definitions:
Client Systems
Client systems refer to the individuals, families, groups, organizations, or communities that are the focus of professional intervention or service provision.
Client Capacities
The abilities, strengths, and resources that clients bring to the therapeutic or service delivery process.
Social Networks
Platforms or structures that facilitate the creation and sharing of information, ideas, personal messages, and other content via virtual communities and networks.
Evaluation
The systematic assessment of the worth or effectiveness of something, typically conducted to inform decision-making.
Q3: In the reaction given, how many grams
Q5: Answer the following questions:<br>a. Briefly explain why
Q15: Which group includes only endothermic processes?<br>A) freezing,
Q25: <sup>14</sup>O, <sup>16</sup>O, <sup>18</sup>O are called _.
Q29: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q30: Calculate the number of grams in 0.928
Q40: A reaction cannot change between being
Q43: Which of the following is not a
Q51: Which of the following best describes an
Q56: Which of the following factors increases the