Answer the following questions:
a. Explain why the solubility of carbonates and phosphates is increased by lowering the pH, but the solubility of chlorides is unaffected by lowering the pH.
b. The solubility of copper(II) carbonate is dramatically increased by the addition of NH3, but the solubility of copper(II) phosphate is not. Referring to the data below, explain why.
Ksp for copper(II) carbonate = 2.5 ´ 10-10
Ksp for copper(II) phosphate = 1.4 ´ 10-37
Cu2+(aq) + 4 NH3(aq)
(Kf = 1.1 ´ 1013)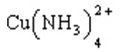
(aq)
Definitions:
Mass Customization
A manufacturing approach that combines the flexibility and personalization of custom-made products with the low unit costs of mass production.
Employee Relationship
The interaction and communication between an employer and its employees, which can significantly impact job satisfaction, productivity, and retention.
HR Practice
Encompasses the policies, procedures, and activities implemented by an organization to manage its human resources effectively.
External Human Resource Sources
External methods or channels through which organizations recruit or hire new employees, such as job boards or recruitment agencies.
Q9: All elements in the Periodic Table beyond
Q12: Calcium carbonate decomposes upon heating to form
Q19: An element containing 26 protons, 26 electrons
Q34: Molarity is a unit of solution concentration
Q35: A weak acid is 5% ionized at
Q37: The equilibrium constant for a particular chemical
Q40: The standard enthalpies of formation for several
Q44: Based solely on the fact that, for
Q46: According to band theory, a semiconductor is
Q48: Explain, with reference to the Nernst equation,