An aqueous solution of phosphoric acid, H3PO4, contains 285 g H3PO4 in 400 mL solution, and has a density of 1.35 g/mL. Calculate
a. the weight % 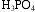 in this solution.
in this solution.
b. The concentration in mol/L of this solution.
Definitions:
Social Loafing
The inclination of people to put forth less effort when they are part of a team as opposed to when they are working by themselves.
Pre-med Requirement
Pre-med requirement encompasses the courses and exams that need to be completed before applying to medical school.
Class Project
A collaborative educational task where students work together in groups to achieve a common goal or complete a specific assignment.
Group Polarization
The tendency of groups to make more extreme decisions than what their members first considered.
Q12: Which choice is an example of a
Q18: A heterogeneous reaction mixture may contain<br>A) only
Q19: Which statement about metallic bonding is not
Q19: What volume of 0.1060 M NaOH is
Q22: Which description of the properties of water
Q28: Which of the following are constitutional isomers?<br>A)
Q35: When was the first period of one-party
Q40: The chemical compound (CH<sub>3</sub>)<sub>3</sub>COH can also be
Q60: Which of the following is correct?<br>A) (<sup>81</sup>Br)
Q64: What is the correct general formula for