Consider the equilibrium reaction 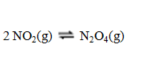 A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g) . What is the value of Kc?
A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g) . What is the value of Kc?
Definitions:
Annual Company Picnic
A social event held by a company for its employees and often their families, typically outdoors and once a year, to foster community and morale.
Mildly Disappointing Information
Information that falls slightly below expectations but is not severely negative or damaging.
Strong Negative Information
Information that is decidedly unfavorable or adverse, often impacting perceptions, decisions, or outcomes significantly.
Indirect Approach
A method of communication or action that is not straightforward, often involving suggestion rather than direct instruction.
Q5: John is upset that the power company
Q9: How many millimeters are in 0.457 yards?<br>A)
Q10: List the steps in the policy-making cycle.Are
Q20: The total number of congressmen and senators
Q20: Which of the following is NOT among
Q23: If the equilibrium constants for the two
Q26: Which of the following would require the
Q29: What factors make it so difficult for
Q41: The study of the relationships between electron
Q56: If a catalyst is added to a