Consider the reaction
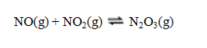 for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
The expression for 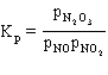 .
.
Definitions:
Weekly Interest Rate
The interest rate applied to a loan or savings, calculated and compounded on a weekly basis.
Average Daily Receipts
The average amount of cash received by a business each day over a specific period, often used to gauge the company's liquidity and cash flow efficiency.
Collection Delay
The time lag between when a company issues an invoice and when it actually receives payment from the customer.
Customers
Individuals or organizations that purchase goods or services from a business, thereby generating revenue for the company.
Q7: Having a large number of interest groups
Q16: In how many different pairs can these
Q20: Which group was the primary target of
Q22: Which characteristic below best fits the description
Q24: The phenomenon of radioactivity was first observed
Q24: Does the metric system of measurement have
Q29: When the reaction shown below is balanced,
Q35: Converting 6.390 pounds to grams yields:<br>A) 2516
Q53: In a neutral polyprotic acid, the value
Q58: Exhibit 12-2 For the following question(s), consider