The rate constant for the reaction 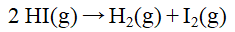 1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, is
1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, is
Definitions:
Standard Error
Indicates the standard deviation of the sampling distribution of a statistic, most commonly the mean.
Standard Deviation
A quantification of how much a group of values diverges or spreads out.
Confidence Interval
An expanse of measurable outcomes, derived from sample analysis, foreseen to incorporate the hidden value of a population attribute.
Standard Deviation
A measure of the amount of variation or dispersion of a set of values from their mean.
Q1: Consider the statement, "At equilibrium, a reaction
Q2: Which is not true of the standard
Q4: If, at constant temperature, the volume of
Q4: List the six categories state policymakers use
Q10: Define the terms "capture" and "iron triangle."
Q16: In how many different pairs can these
Q30: What is the largest single contributor to
Q37: During the time of the Republic of
Q40: A reaction cannot change between being
Q50: Typically, for a weak acid and its