What volume (in L) of oxygen at 298K and 1.50 atm is required for the complete combustion of 25.0 g of hexane? The value of R = 0.0821 L atm mol-1 K-1. 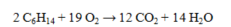
Definitions:
Agenda Setting
The ability of the news media and public policy makers to influence the importance placed on topics of the public agenda.
Public Opinion
The aggregate of individual attitudes or beliefs held by the adult population.
Gallup Poll
A statistical survey that measures public opinion, conducted by the Gallup Organization.
News Media
Organizations and platforms that gather, produce, and distribute news to the public, including newspapers, television, radio, and the internet.
Q1: What is the most important difference between
Q15: Which state of matter has no definite
Q17: Consider the endothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q27: In Texas,the state budget is submitted by<br>A)
Q28: Which unit allows for the varying effects
Q30: What is the most common form of
Q39: At a particular temperature, a reactant-favored process
Q41: Which statement about relative humidity is false?<br>A)
Q48: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q64: An enzyme is a(n)<br>A) organic or inorganic