Determine the volume (in L) of Cl2(g) required to carry out the following reaction at 794 torr and 625°C using 15.0 g of Fe. The value of R = 0.0821 L atm mol-1 K-1. 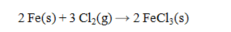
Definitions:
Redefines
To assign a new definition or meaning to an existing term or concept, often in the context of programming or methodologies.
Method Print
A function in programming languages used to output or display data to a standard output device.
Instanceof Operator
An operator used in Java to check whether an object is an instance of a specific class or an interface.
Correct Syntax
The set of rules that defines the combinations of symbols that are considered to be correctly structured programs or expressions in a programming language.
Q3: The lowest number of states select judges
Q13: What is the composition of the county
Q13: Which of the following is a rational
Q20: Discuss tax shifting,and cite specific examples showing
Q21: Once the reaction quotient, Q, has been
Q37: The rate of the chemical reaction involving
Q40: The table below gives the rate constant
Q45: Which of the following factors increases the
Q49: Balance the following redox equation.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"
Q51: Which statement about ideal gases and the