What volume (in L) of oxygen at 298K and 1.50 atm is required for the complete combustion of 25.0 g of hexane? The value of R = 0.0821 L atm mol-1 K-1. 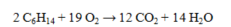
Definitions:
Operant Conditioning
A method of learning that occurs through rewards and punishments for behavior, emphasizing the influence of consequences on behavior.
Shaping
A method of operant conditioning by which successive approximations toward a desired behavior are reinforced.
Conditioned Stimulus
A stimulus that initially has no inherent value but acquires significance and the ability to elicit a response through its association with an unconditioned stimulus.
Unconditioned Stimulus
In classical conditioning, a stimulus that naturally and automatically triggers an unconditioned response without prior learning.
Q11: In recent years,most of the growth in
Q14: Which statement is correct?<br>A) all electrolytic cells
Q18: An element which has some properties of
Q20: Discuss tax shifting,and cite specific examples showing
Q21: The merit system used to identify and
Q27: Drinkable water can be produced from seawater
Q32: Which is not a greenhouse gas?<br>A) carbon
Q32: Discuss how money is an especially important
Q38: Which of the following can help offset
Q59: Which characteristics apply to the gaseous state?<br>I.