Based on the following electrochemical cell, which statement is ? 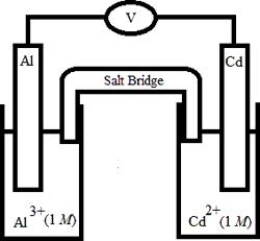 Half-Reaction E° (V) Al3+(aq) + 3e- →
Half-Reaction E° (V) Al3+(aq) + 3e- →
−1) 66
Al(s)
Cd2+(aq) + 2e- →
−0) 40
Cd(s)
Definitions:
Endowment Management
The process of overseeing and making investment decisions for an endowment fund to ensure its ability to support a particular organization or cause over time.
Bookkeeping
The practice of recording all financial transactions, ensuring accuracy and completeness in the financial records of an organization.
Accrual Basis Accounting
An accounting method where income and expenses are recorded when they are earned or incurred, regardless of when the cash is actually received or paid.
Cash Flow
The total amount of money being transferred into and out of a business, affecting the organization's liquidity.
Q2: What is the density of CO<sub>2</sub>(g) at
Q21: Identify the missing species in the following
Q23: Which one of these structures represents a
Q31: What is the name of a proton
Q50: What is the conjugate acid of the
Q61: Which is a correct description of the
Q112: Two commonly used techniques to aid mangers
Q136: In 25 words of less, write a
Q285: Based on Figure 2-5 above, identify and
Q314: According to Figure 2-3b above, which of