At 25°C, the following heats of reaction are known: 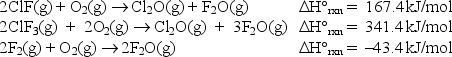 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
Definitions:
CSI Television Series
A popular American TV show that follows crime scene investigators as they solve crimes through forensic science.
DNA
Deoxyribonucleic acid, a molecule that carries the genetic instructions used in the growth, development, functioning, and reproduction of all living organisms and many viruses.
SOC Code
An acronym for Standard Occupational Classification code, which is a system used to classify workers into occupational categories for the purpose of collecting, calculating, or disseminating data.
Major Group
A classification system used to group similar occupations or academic fields for organizational or analytical purposes.
Q4: How many unpaired electrons does a ground-state
Q27: Balance the following chemical equation:<br>NH<sub>3</sub> +
Q33: The bond angle in Cl<sub>2</sub>O is expected
Q48: A gaseous compound is 30.4% nitrogen and
Q49: Which of the bonds below would have
Q66: Which of the following compounds is a
Q78: Write the ground state electron configuration for
Q92: According to the VSEPR theory, the molecular
Q152: The mass of 1.63 * 10<sup>21</sup> silicon
Q154: Nickel has a lower atomic mass than