Use the Born-Haber cycle to calculate the lattice energy of KCl(s) given the following data: H(sublimation) K = 79.2 kJ/mol
I1 (K) = 418.7 kJ/mol
Bond energy (Cl-Cl) = 242.8 kJ/mol
EA (Cl) = 348 kJ/mol
H 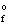 (KCl(s) ) = -435.7 kJ/mol
(KCl(s) ) = -435.7 kJ/mol
Definitions:
Variance
A statistical measure of the dispersion of a set of data points in relation to their mean.
Mean
The arithmetic average of a set of numbers, calculated by adding all the numbers together and dividing by the count of numbers.
Variance
A measure of the dispersion or spread of a set of data points around their mean value.
Degrees of Freedom
The number of independent values or quantities that can vary in the calculation of a statistic, often necessary for assessing the significance of the statistic.
Q9: The electron configuration of a ground-state copper
Q19: What type of chemical bond holds the
Q20: What is the osmotic pressure of a
Q22: Which of the following is not true
Q27: Give the number of lone pairs around
Q48: If a hydrogen atom and a helium
Q60: Arrange the following in order of increasing
Q63: The colors of the visible spectrum are
Q92: Calculate the wavelength associated with a <sup>20</sup>Ne<sup>+</sup>
Q99: The molecular property related to the ease