Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 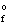 ) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
Definitions:
Teen Years
The period of life that follows the onset of puberty and precedes legal adulthood; generally considered to span from ages 13 to 19.
Male Sex Hormones
Chemical substances produced primarily by the testes in males that are responsible for the development of male sexual characteristics and reproductive functions; testosterone is a primary example.
Intersex Development
A condition in which an individual is born with reproductive or sexual anatomy that doesn’t fit typical definitions of male or female.
Transgender
A term describing someone whose gender identity differs from the sex they were assigned at birth.
Q7: Which of the following substance is/are planar?
Q8: Which of the following aqueous solutions has
Q23: The successive ionization energies of a certain
Q23: What is the molarity of a solution
Q26: Calculate the standard enthalpy change for
Q30: A block of dry ice (solid CO<sub>2</sub>,
Q38: Which of the following elements has the
Q51: Consider the species O<sub>2</sub><sup>-</sup>, O<sub>2</sub>, and O<sub>2</sub><sup>+</sup>.
Q75: Given that CaO(s)+ H<sub>2</sub>O(l) <span class="ql-formula"
Q104: Which of the following processes is