For the chemical reaction system described by the diagram below, which statement is true? 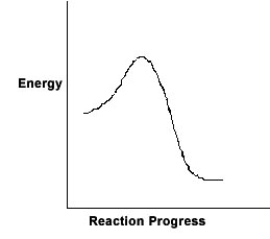
Definitions:
Health Promotion
Activities and practices that enhance well-being and improve health outcomes through the encouragement of healthy behaviors.
Influenza Vaccination
The act of receiving a vaccine to protect against the influenza virus, recommended annually to reduce the risk of flu and its complications.
Cardiac Rehabilitation
A customized program of exercise, education, and support to help patients recover after a heart attack, surgery, or other heart conditions.
Macrobiotic, Vegetarian
A dietary regimen emphasizing whole grains, vegetables, and beans, while avoiding processed foods and meats, to promote health and longevity.
Q2: The reaction A + 2B
Q18: Find the temperature at which the
Q27: Sodium carbonate can be made by
Q36: What phase exists at the point labeled
Q43: The Arrhenius equation is k = Ae<sup>-Ea/RT</sup>.
Q52: Indicate all the types of intermolecular forces
Q54: An environmental chemist obtained a 200. mL
Q57: The expected product from the addition of
Q66: Potassium bromide, KBr, crystallizes like NaCl in
Q97: The pH of a 0.6 M solution