A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq) 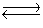 Cd(CN)42-(aq)Kc = 1.0 * 1025
Cd(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of CN-(aq)at equilibrium?
Definitions:
Visual Acuity
The clarity or sharpness of vision, often measured by the ability to discern letters or numbers at a standardized distance on an eye chart.
Conjunctivitis
An inflammation or infection of the outer membrane of the eyeball and the inner eyelid, also known as pink eye.
Chalazion
A benign, painless bump or nodule inside the upper or lower eyelid caused by inflammation of a meibomian gland.
Pinkeye
A common term for conjunctivitis, an inflammation or infection of the outer membrane of the eyeball and the inner eyelid.
Q7: A 0.10 M NH<sub>3</sub> solution is
Q17: What volume of water should be added
Q32: Which species will have the greatest absolute
Q61: NaCl is added slowly to a solution
Q63: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according
Q73: The number of atoms in a face-centered
Q81: The isomerization of cyclopropane to form propene
Q95: Given that E<sub>a</sub> for a certain biological
Q108: Dissolving an ionic solid in water always
Q138: Given the following compound and its boiling