Two moles of PCl5 are placed in a 5.0 L container. Dissociation takes place according to the equation PCl5 (g) 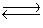 PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
Definitions:
High Negative Affectivity
A personality trait encompassing pervasive mood states of anxiety, depression, and hostility.
External Locus of Control
A belief that one's outcomes or events in life are determined by forces outside one’s control, such as luck or other people's actions.
Workaholics
Workaholics are individuals who compulsively work long hours, well beyond what is required, often to the detriment of personal relationships and health.
Repressed Anger
Anger that is suppressed and not consciously acknowledged, which can lead to psychological and emotional issues if not addressed.
Q7: Which is the correct equilibrium constant expression
Q15: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3244/.jpg"
Q18: For what order reaction does the half-life
Q27: The activation energy for the reaction
Q33: Which one of these salts will form
Q37: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to sulfur
Q87: The equilibrium constant for the reaction
Q100: An experimental drug, D, is known to
Q106: For a given substance the entropy always
Q126: What mass of sodium formate (HCOONa)must be