Consider the two gaseous equilibria: SO2(g) + 1/2O2(g) 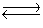 SO3(g) K1
SO3(g) K1
2SO3(g) 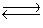 SO2(g) + O2(g) K2
SO2(g) + O2(g) K2
The values of the equilibrium constants K1 and K2 are related by
Definitions:
Rationalizing The Theft
involves justifying or providing logical reasons to legitimize the act of theft, often to alleviate guilt or to normalize the behavior.
Auto Thefts
The act of illegally taking possession of someone else's vehicle with the intent to permanently keep it.
Inadequate Law Enforcement
A situation where the law enforcement agencies are unable or fail to effectively enforce laws, leading to issues like increased crime and public distrust.
Desire To Use Violence
An inclination or urge to engage in physical force to achieve a goal or express emotions.
Q31: Suppose the atoms in a two-dimensional crystal
Q39: What volume of 0.200 M potassium hydroxide
Q43: A solution is prepared by adding 40.3
Q49: The systematic name for the hydrocarbon with
Q57: If the pH of stomach acid is
Q66: Write the chemical formula for the acid
Q72: Which of these situations will result if
Q112: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g)
Q113: Which of the following is not an
Q118: Consider an electrochemical cell constructed from the