For the reaction at equilibrium 2SO3 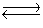 2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
Definitions:
Dollar Amount
A specific value or quantity of money expressed in the currency of the United States.
Investment Company
A corporation or trust engaged in the business of investing the pooled capital of investors in financial securities, including stocks, bonds, and money market instruments.
Intermediary
An entity that acts as a middleman between two parties in a financial transaction.
Pools
Aggregated funds from multiple investors used to buy securities, real estate, or other investments as a collective group.
Q6: MgO has the same crystal structure as
Q17: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q23: The group of atoms that is responsible
Q25: Some KCl is dissolved in water 25°C,
Q34: Which one of the following substances is
Q50: When the concentrations of reactant molecules are
Q53: Using the thermodynamic data provided below, calculate
Q89: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>-</sup>+
Q101: Choose the substance with the higher entropy
Q148: In comparing three solutions with pH's of