A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq) 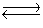 Cu(CN)42-(aq)Kc = 1.0 * 1025
Cu(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of Cu2+(aq)at equilibrium?
Definitions:
Number of Siblings
The count of brothers and sisters an individual has.
Type I Error
Wrongly dismissing a true null hypothesis, commonly called a "false positive."
Type II Error
The statistical error occurring when a test fails to reject a false null hypothesis, also known as a "false negative."
Type I Error
The mistake of rejecting a true null hypothesis, also known as a false positive.
Q26: Which of the following is not true
Q34: For the reaction X + Y
Q39: How does the entropy change when a
Q48: At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q52: The solubility of gases in water usually
Q73: A solution is prepared by mixing 500.
Q74: The data below were determined for
Q80: Solids cannot react with gases.<br>A)1 and 2<br>B)1
Q91: Use the graph of vapor pressure to
Q120: Explain why CO<sub>2</sub> is nonpolar, but OCS