A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq) 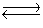 Cd(CN)42-(aq)Kc = 1.0 * 1025
Cd(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of CN-(aq)at equilibrium?
Definitions:
Shortest Period
Typically refers to the briefest duration or smallest amount of time required for an event or condition to complete or occur.
Sensory
Pertaining to the senses or sensation, involving the reception and transmission of signals to the brain.
Short-Term
Referring to a limited period of time, often contrasting with long-term or permanent situations.
Long-Term
Referring to an extended period of time, often implying durability or sustained attention beyond the short-term or immediate future.
Q1: Write the formula for the alcohol and
Q10: A 9.50 % by mass solution of
Q15: Which one of the following reagents is
Q52: Determine the equilibrium constant (K<sub>p</sub>)at 25°C
Q83: An aqueous dextrose solution having a density
Q89: Platinum has a face-centered cubic crystal structure
Q102: Consider the following standard reduction potentials in
Q106: Choose the response that lists the member
Q111: Nitrogen pentoxide decomposes by a first-order
Q124: Which of the following properties indicates the