Consider the following equilibrium,
4NH3(g)+ 3O2(g) 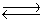 2N2(g)+ 6H2O(g)+ 1531 kJ
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant when the temperature is increased.
Definitions:
Equilibrium Price
The price at which the quantity of a product demanded by consumers equals the quantity supplied by producers, leading to market stability.
Equilibrium Quantity
The amount of products or services available that matches the amount requested at the price where supply and demand balance.
Supply Increases
A situation where the quantity of a good or service that producers are willing to supply at a certain price rises.
Equilibrium Price
The market price where the quantity of goods supplied is equal to the quantity of goods demanded.
Q14: For the chemical reaction A
Q27: You have 500.0 mL of a buffer
Q45: To 1.00 L of a 0.100 M
Q52: Complete and balance the following redox
Q61: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3244/.jpg"
Q62: For the reaction H<sub>2</sub>(g)+ S(s)
Q72: Which of the following has the greater
Q81: Will a precipitate (ppt)form when 300. mL
Q83: At 400ºC, K<sub>c</sub> = 64 for the
Q115: In aqueous solutions at 25°C, the sum