Kc for the reaction CO2(g)+ H2(g) 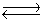 H2O(g)+ CO(g)is 1.6 at about 990ºC. Calculate the number of moles of hydrogen gas in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
H2O(g)+ CO(g)is 1.6 at about 990ºC. Calculate the number of moles of hydrogen gas in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Write Off
An accounting action resulting in the reduction of the book value of an asset due to uncollectibility or loss of value, impacting profit and loss statements.
Accounting Equation
The fundamental equation that represents the relationship between a company's assets, liabilities, and shareholders' equity: Assets = Liabilities + Stockholders' Equity.
Note Receivable
A note receivable is a financial document representing a written promise for payment received by one party from another, typically including specifics of interest rate and maturity date.
Payment Receipt
A document that serves as proof of a transaction between two parties, indicating that payment has been made and received.
Q22: The isomerization of cyclopropane follows first order
Q27: Which one of these hydrocarbons does not
Q41: The vapor pressure of a liquid in
Q48: Find the emf of the cell described
Q56: Which of these is the systematic name
Q62: Complete and balance the following redox
Q65: The oxidation of iodide ions by
Q67: Consider the following electrochemical cell: U |
Q78: Solid ammonium hydrogen sulfide is introduced into
Q90: Calculate the equilibrium constant for the