Short Answer
Kc for the reaction CO2(g)+ H2(g) 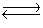 H2O(g)+ CO(g)is 1.6 at about 990ºC. Calculate the number of moles of water in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
H2O(g)+ CO(g)is 1.6 at about 990ºC. Calculate the number of moles of water in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Differentiate among the genetic, biological, socioemotional, and cognitive aspects of development.
Learn the sequence of developmental periods from prenatal to adolescence.
Recognize the role of early and later experiences in child development.
Understand the scientific approach in developmental psychology.
Definitions:
Related Questions
Q4: Which one of the following reagents is
Q15: The rate law predicted by the
Q17: A certain first-order reaction A
Q56: A titration of an acid and base
Q57: The expected product from the addition of
Q85: Explain the following, on the basis of
Q96: Which of the following substances would have
Q111: Which one of these salts will form
Q133: Complete and balance the following redox
Q144: What is the pH of a 0.014