Kc for the reaction CO2(g)+ H2(g) 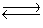 H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of carbon monoxide in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of carbon monoxide in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Modified Leaf
A leaf that has evolved to serve functions other than photosynthesis, such as protection or reproduction.
Bulb
A globose, fleshy, underground bud that consists of a short stem with fleshy leaves (e.g., onion).
Leaf Abscission
The natural process by which leaves detach from a plant, typically in response to environmental triggers or seasonal changes.
Abscisic Acid
A plant hormone that regulates many aspects of plant growth and development, including stress responses, seed dormancy, and stomatal closure.
Q7: What is the osmotic pressure of a
Q12: The octane rating of gasoline refers to
Q20: A first-order reaction has a rate constant
Q30: Which one of the following substances is
Q33: The equilibrium constant for the reaction
Q36: A solution of carbon tetrachloride in benzene,
Q37: Which functional group, when present in a
Q75: Which of these metals will be oxidized
Q95: The concentration of nitrogen in water at
Q122: Complete and balance the following redox