Two moles of PCl5 are placed in a 5.0 L container. Dissociation takes place according to the equation PCl5 (g) 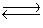 PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
Definitions:
Emergency Supplies
Items and resources stored in preparation for unexpected events or disasters to ensure survival and safety.
Strategic Marketing Process
A methodical approach to planning and executing marketing strategies to achieve business objectives.
Marketing Program
A coherent and structured set of marketing strategies and activities aimed at achieving specific business goals.
Organization
An entity comprising multiple people working together towards a common goal, which can be classified as businesses, government bodies, or not-for-profits.
Q4: The oxidation of iodide ions by
Q14: For the reaction Ni<sup>2+</sup>(aq)+ 2Fe<sup>2+</sup>(aq) <span
Q16: Which one of the following reactions
Q42: A compound with the formula C<sub>6</sub>H<sub>12</sub> may
Q63: Will a precipitate of magnesium fluoride form
Q86: Predict the direction in which the
Q95: The overall reaction 2Co<sup>3+</sup>(aq)+ 2Cl<sup>-</sup>(aq) <span
Q102: Calculate the H<sup>+</sup> ion concentration in a
Q124: Which of the following properties indicates the
Q130: For the reaction, 2Cr<sup>2+</sup> + Cl<sub>2</sub>(g)