Equilibrium constants are known for the following reactions:
S(s)+ 3/2O2(g) 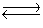 SO3(g)Kc = 9.2 * 1023
SO3(g)Kc = 9.2 * 1023
SO3(g) 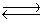 SO2(g)+ 1/2O2(g)Kc = 4.8 * 10- 4
SO2(g)+ 1/2O2(g)Kc = 4.8 * 10- 4
Thus, for the reaction S(s)+ O2(g) SO2(g), Kc = 4.4 * 1020.
Definitions:
Distribution Of Scores
A representation, often in graphical form, showing how frequently different scores appear in a set of data.
Arithmetic Average
The central value of a set of numbers, calculated by dividing the sum of all the numbers by the count of those numbers.
Arithmetic Average
The sum of a collection of numbers divided by the count of numbers in the collection, commonly known as the mean.
Extreme Cases
Situations or examples that are highly unusual or atypical, often used to test the limits of theories or understanding.
Q22: Iron objects such as storage tanks and
Q27: What is the molarity of a solution
Q36: What phase exists at the point labeled
Q52: K<sub>p</sub> for the reaction of SO<sub>2</sub>(g)with O<sub>2</sub>
Q55: How many moles of silver metal are
Q79: Which one of the following elements would
Q80: Solids cannot react with gases.<br>A)1 and 2<br>B)1
Q103: The solubility of a solid always increases
Q131: Which is not a characteristic property of
Q135: Aluminum metal is formed by the electrolysis