Calculate G° for the reaction 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) . 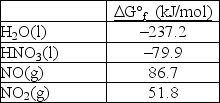
Definitions:
Yen
The official currency of Japan, known internationally by its symbol ¥.
Deadweight Loss
A loss of economic efficiency that can occur when equilibrium is not achievable or not achieved in a market.
Subsidy
A financial contribution granted by the government or a public body to support businesses or consumers, making goods or services more affordable.
Manure
Organic matter, mainly derived from animal feces, used as fertilizer in agriculture.
Q2: In the complex ion [Fe(CN)<sub>6</sub>]<sup>4-</sup>, the oxidation
Q18: How many coulombs of charge are required
Q34: Polystyrene results from the polymerization of <img
Q46: If one starts with pure NO<sub>2</sub>(g)at a
Q57: Find the nuclear binding energy of potassium-40
Q62: Hard water deposits (calcium carbonate)have built up
Q69: How long will it take to produce
Q85: Determine the equilibrium constant (K<sub>eq</sub>)at 25°C
Q98: Determine the equilibrium constant, K<sub>eq</sub>, at
Q152: An aqueous solution of KCl would be<br>A)neutral.<br>B)basic.<br>C)acidic.