Calculate G° for the combustion of ethanol vapor, C2H5OH(g) , at 750°C in oxygen to form carbon dioxide and water vapor. The following data is valid at 25°C: 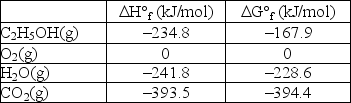
Definitions:
Alteration Defense
A legal argument used when a document has been changed or modified, affecting the obligations or rights under that document.
Presentment
The formal presentation of a document such as a check or bill of exchange for payment or acceptance.
Negotiable Instrument
A document that ensures the payment of a specified sum of money, either upon request or at an established date, with the payer's name indicated on it.
Presentment Warranties
Guarantees made by the presenter of a negotiable instrument, such as a check, regarding the legitimacy and authority to transfer the instrument.
Q8: What conditions are used in the Haber
Q10: Aluminum does not corrode as does iron,
Q20: Which one of these materials is a
Q25: Find the concentration of Pb<sup>2+</sup> ions in
Q27: Which of the following is an advantage
Q43: A company whose inventory turnover ratio is
Q96: What is the free energy change
Q97: What would the atom ratio of <sup>206</sup>Pb
Q108: At a particular temperature the first-order
Q134: Consider an electrochemical cell involving the