Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 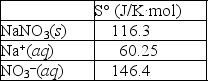 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Definitions:
Ethical Cultures
The collective ethos and ethical orientation that characterize the environment of a business organization, influencing its decision-making and relationships.
Power Distance
A measure of how power is distributed and the extent of acceptance of unequal power distribution within a society or organization.
Auditing Process
A systematic examination and evaluation of an organization's financial statements, processes, or systems to ensure accuracy, compliance, and integrity.
Triple Bottom Line
An accounting framework that goes beyond the traditional measures of profit, return on investment, and shareholder value to include environmental and social dimensions.
Q5: Strontium-90 has a half-life of 28.8 years.
Q5: The equilibrium between carbon dioxide gas and
Q8: Write an equation showing the net reaction
Q9: The following reaction is spontaneous under
Q10: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q19: The half-reaction that should occur at
Q39: The equilibrium constant for the reaction
Q66: Write the chemical formula for the acid
Q104: The OH<sup>-</sup> concentration in a 2.5 *
Q271: Which one of the following statements about