Calculate G° for the reaction 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) . 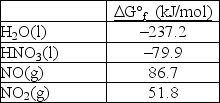
Definitions:
Wage Rate
The amount of money paid to an employee per unit of time or piece of work completed.
Leisure
Time spent away from work and essential domestic activities, often used for rest, recreation, or cultural pursuits.
Inferior Good
A type of good for which demand decreases when consumer income rises, in contrast to normal goods, where demand increases with rising income.
Consumer Income
The total amount of income earned by consumers in an economy, including wages, salaries, benefits, and other income sources, influencing their buying power.
Q14: The backbone of a strand of nucleic
Q27: Given the following standard reduction potentials
Q39: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3244/.jpg"
Q48: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q52: Calculate the concentration of fluoride ions in
Q53: The data below refer to the following
Q63: The radioisotope potassium-40 decays to argon-40 by
Q81: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q84: The solubility product constant at 25°C
Q107: When an aqueous solution of NaCl is