Calculate G° for the combustion of ethanol vapor, C2H5OH(g) , at 750°C in oxygen to form carbon dioxide and water vapor. The following data is valid at 25°C: 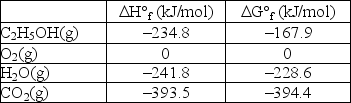
Definitions:
Productivity
The measure of efficiency of a person, machine, factory, system, etc., in converting inputs into useful outputs.
Scientific Management
A theory of management that analyzes and synthesizes workflows, aiming to improve economic efficiency, especially labor productivity, by scientific means.
Labor Efficiency
A measure of produced output by a labor unit or units in a defined time period, indicating productivity.
Psychological Growth
Refers to the development and maturation of an individual's mind, character, and ability to cope with and understand their emotions and the emotions of others.
Q3: Which of these species has the highest
Q8: Melting an ionic solid always results in
Q20: A spontaneous endothermic reaction always<br>A)causes the surroundings
Q44: Ethanol and acetic acid react to form
Q49: Calculate the hydrogen ion concentration in a
Q61: Will a 0.1 M solution of NH<sub>4</sub>NO<sub>2</sub>(aq)be
Q78: Calculate the H<sup>+</sup> ion concentration in a
Q91: Find the nuclear binding enrgy of uranium-234
Q93: Assuming <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q96: Identify the conjugate acid of HCO<sub>3</sub><sup>-</sup>