Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 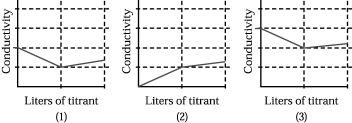
-Which of the above graphs corresponds to titration I?
Definitions:
Service Selection
The process of choosing among various service providers or service offerings based on criteria such as quality and price.
Choiceboard
A digital platform that enables customers to personalize products or services based on their own choices.
Electronic Dialog
The communication that occurs through electronic means, such as emails, chat rooms, and social media platforms, between businesses and customers.
Customerization
A blend of customization and standardization in marketing, where businesses adapt their products or services to individual customer needs while maintaining efficiency.
Q51: An orbital that as the appearance of
Q109: The element in period 3 with the
Q114: Which is expected to have the strongest
Q117: The reaction 2 HNO<sub>3</sub>(aq)+ Ba(OH)<sub>2</sub>(aq)→ Ba(NO<sub>3</sub>)<sub>2</sub>(aq)+ 2
Q146: Which contains the greatest number of chloride
Q149: How many valence shell electrons does an
Q150: Which of the following represents the change
Q151: Which of the following have their valence
Q166: A student dissolved 4.00 g of Co(NO<sub>3</sub>)<sub>2</sub>
Q176: The compound,NO<sub>2</sub>,is named<br>A)nitrate.<br>B)nitrite.<br>C)nitrogen dioxide.<br>D)nitrogen(IV)oxide.