Which group of elements,indicated by letter on the periodic table,has electrons with the ground-state valence-shell electron configuration ns2 np4? 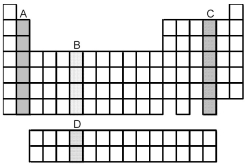
Definitions:
Strategic Financial
Involves the management of a company's finances with the aim to achieve its short-term and long-term goals using strategic planning and analysis.
Tenants in Common
A form of co-ownership where two or more individuals hold ownership rights to a property, with each owner having a divisible interest that can be transferred independently.
Jointly Controlled Assets
Those assets that are owned and operated under a joint agreement by two or more parties, where the parties have control over the asset and share in any resultant outcomes.
Proportionate Interest
Refers to an investor's share of profits, losses, and assets in a joint venture or partnership, reflecting their ownership percentage.
Q46: The laser used to read Blu-Ray discs
Q48: The number of grams of NaCl required
Q55: What is the third-row element having the
Q85: A quantized variable<br>A)can be continuously varied.<br>B)can only
Q113: Which of the following most likely represent
Q124: Write a balanced net ionic equation for
Q127: List all the elements that have a
Q148: What reagent could not be used to
Q156: The Lewis electron-dot structure of N<sub>2</sub> has
Q199: What is the bond angle in the