Calculate the energy change for the formation of LiCl(s) from its elements in their standard states and the following tabulated information: 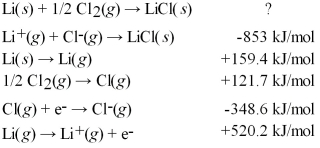
Definitions:
Certified Nurse Practitioner
A registered nurse who has advanced education and training in a particular area of healthcare, authorized to perform tasks beyond the scope of registered nursing.
Teamwork and Collaboration
The coordinated effort of a group of individuals working together towards a common goal, often found in healthcare settings to improve patient outcomes.
Float
In a nursing or hospital context, to move or be assigned to different wards or units as needed, rather than having a fixed place.
Oncology Unit
A specialized department within a hospital or medical facility that provides treatment and care for cancer patients.
Q4: If the density of ethanol,C<sub>2</sub>H<sub>5</sub>OH,is 0.789 g/mL.How
Q5: The hybrid orbital used by nitrogen to
Q17: What is the bond angle in the
Q25: What is the stoichiometric coefficient for oxygen
Q39: The first vibrational level for NaH lies
Q76: The greater the energy of a photon,the<br>A)longer
Q79: Of H<sub>2</sub>CO and CO and CO<sub>2</sub>,the compound
Q98: What is the smallest bond angle in
Q122: Which picture corresponds to potassium fluoride?<br>A)picture (a)<br>B)picture
Q130: Which of the following ionic compounds would