Multiple Choice
Calculate the energy change for the formation of LiCl(s) from its elements in their standard states and the following tabulated information: 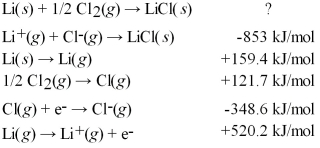
Definitions:
Related Questions
Q5: For a particular process that is carried
Q12: Among the compounds H<sub>3</sub>C-CH<sub>3</sub>,H<sub>2</sub>C=CH<sub>2</sub>,and HC=CH,the compound with
Q20: Dinitrogen monoxide gas decomposes to form nitrogen
Q62: What is the name for the group
Q83: When carbon dioxide dissolves in water,H+ is
Q114: Which is expected to have the strongest
Q121: Photochemists use electromagnetic radiation to initiate chemical
Q161: In the reaction of sodium metal with
Q207: The balanced equation for the decomposition of
Q229: Of XeF<sub>2 </sub>and XeF<sub>4,</sub>the one with the