Calculate the energy change for the formation of CaF2(s) from its elements in their standard states and the following information: 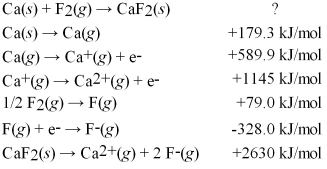
Definitions:
Pretreatment State
The condition or state of a patient before undergoing a therapeutic intervention or treatment.
DBT
Dialectical Behavior Therapy, a comprehensive cognitive-behavioral treatment that emphasizes individual psychotherapy and group skills training classes to help people learn and use new skills and strategies to develop a life that they experience as worth living.
Dialectic Tension
The conflict or tension that arises from two opposing or incompatible forces, ideas, or desires.
DBT Therapist
A clinician trained in Dialectical Behavior Therapy, a type of cognitive-behavioral therapy that combines standard cognitive-behavioral techniques for emotion regulation with concepts of distress tolerance, mindfulness, and acceptance.
Q2: What is the oxidation number of the
Q24: CH<sub>3</sub>CO<sub>2</sub>H is an example of a _
Q53: What is expected when the reaction shown
Q82: What is the likely formula for the
Q83: Calculate the energy change for the formation
Q97: The element in period 4 with the
Q101: When dissolved in water,KOH behaves as<br>A)an acid
Q115: What is the ground-state electron configuration of
Q156: The Lewis electron-dot structure of N<sub>2</sub> has
Q166: A student dissolved 4.00 g of Co(NO<sub>3</sub>)<sub>2</sub>