Multiple Choice
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 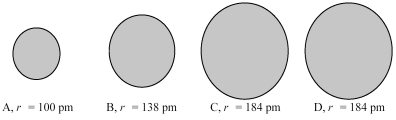
-Which sphere most likely represents the K+ ion?
Definitions:
Related Questions
Q25: For the fourth-shell orbital shown below,what are
Q33: Which of the following is not true?<br>A)All
Q44: Which ionization process requires the most energy?<br>A)W(g)→
Q51: Phthalic acid is a diprotic acid having
Q53: What is geometry around the carbon atom
Q54: Predict the product(s)of the reaction of Br<sub>2</sub>(aq)with
Q82: Molybdenum has an anomalous electron configuration.Write the
Q133: Atoms of which element,indicated by letter on
Q196: Using only the elements Ba,Cl,and P,give the
Q197: Arrange in order from the smallest to