Multiple Choice
Atoms of which element,indicated by letter on the periodic table,would be expected to have the most negative value of Eea? 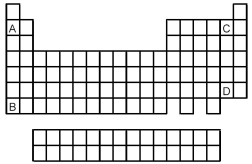
Definitions:
Related Questions
Q26: Based on VSEPR theory,which should have the
Q31: Arrange the following spectral regions in order
Q53: Of the following,which atom has the largest
Q71: Which of the following is not a
Q78: Predict the products of a reaction between
Q83: Calculate the energy change for the formation
Q100: The heat of vaporization of water at
Q115: A molecular compound that obeys the octet
Q151: Calculate ΔG° for the reaction below and
Q190: A chlorine atom in Cl<sub>2</sub> should have