Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 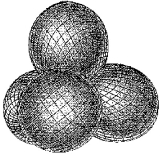
Definitions:
Coupon Rate
The interest rate per year on a bond, expressed as a fraction of its face value.
Par-Value
The face value of a bond or stock, representing the amount that the issuer agrees to repay at maturity or the nominal value of a share, respectively.
Duration
A measure of the sensitivity of a bond's price to changes in interest rates, essentially a weighted average of the time until cash flows are received.
Coupon Bond
A type of bond that pays the holder a fixed interest payment (coupon) at regular intervals until the maturity date, when the principal amount is repaid.
Q75: The heat of vaporization of water at
Q77: Which drawing best shows the approximate level
Q85: The oxidation number of hydrogen in CaH<sub>2</sub>
Q90: Copper has the anomalous electron configuration _.
Q121: At what temperature will sulfur hexafluoride molecules
Q128: To reach a noble gas electron configuration
Q150: For the fourth-shell orbital shown below,what are
Q153: A gas bottle contains 0.650 mol of
Q201: Which is the most acceptable electron dot
Q208: What is the molecular geometry of Te