Imagine a reaction that results in a change in both volume and temperature,as shown in the diagram below.What is the sign of the work being done and the sign of the enthalpy change involved in this reaction? 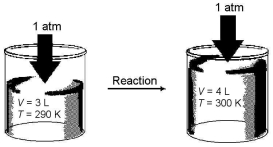
Definitions:
Q30: A reaction that absorbs 49.6 kJ from
Q40: An incorrect statement about the alkaline earth
Q43: The two most efficiently packed unit cells
Q53: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>Cl<sub> </sub><br>B)HBr<sub>
Q84: What is the geometry around the central
Q120: What is the angle between adjacent sp<sup>3</sup>
Q143: The product,Z,is represented by<br>A)arrow A.<br>B)line D.<br>C)line E.<br>D)line
Q153: A gas bottle contains 0.650 mol of
Q156: Step (1)in the reaction is represented by<br>A)arrow
Q178: When using the ideal gas law the