Acetylene torches utilize the following reaction:
2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g)
Use the given standard enthalpies of formation to calculate ΔH° for this reaction. 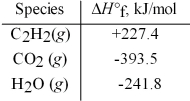
Definitions:
Self-esteem
A person's personal assessment of their self-value or worth.
Role Stress
Psychological stress or tension resulting from conflicting demands, expectations, or roles associated with one's job.
Organizational Commitment
An employee's psychological attachment to their organization, often resulting in a willingness to remain with the organization and go above minimal requirements.
High Self-monitors
Individuals highly sensitive to the social and interpersonal cues in their environment, thus able to adjust their behaviors accordingly to different situations.
Q14: At constant pressure for which of the
Q47: Shown below is a model of CH<sub>4</sub>
Q58: If NO<sub> </sub> and NH<sub>3</sub> are allowed
Q65: Which of the following gases has the
Q80: Which sphere most likely represents the S<sup>2-</sup>
Q89: Which of the following gases has the
Q119: List the elements Cs,Ca,Ne,Na,Ar in order of
Q151: The change in the Gibbs free energy
Q160: _ energy is the kinetic energy of
Q209: The molecular geometry of COCl<sub>2</sub> is _.