The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 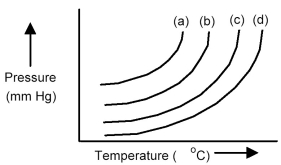
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for diethyl ether?
Definitions:
Pore Spaces
The voids or gaps between soil or rock particles, which can contain water or air.
Water Pressure
The force exerted by water on any surface it is in contact with, significant in various geological and biological processes.
Dewatered Area
describes a region from which water has been removed, typically for construction or drainage purposes.
Upper Level
Refers to the higher layers of the atmosphere or any system, often used in meteorology or geosciences to describe phenomena occurring at altitude.
Q3: Which of the areas designated A,B,and C
Q8: What volume of 0.716 M KBr solution
Q11: Which of the following is not an
Q31: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Q45: If mercury (density = 13.6 g/cm<sup>3</sup>)at a
Q69: Calculate the total quantity of heat required
Q87: For the first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+
Q115: Which best indicates the direction of the
Q147: If an endothermic reaction is spontaneous at
Q180: At 300°C decomposition of NO<sub>2</sub>(g)occurs with a