The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 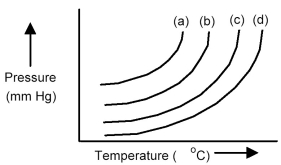
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for ethanol?
Definitions:
Common Stockholders' Equity
Equity representing the ownership interest of common shareholders in a company, reflected as the share capital and retained earnings.
Dividends Paid
The sum of money distributed to shareholders from a company's earnings.
Net Income
The total profit of a business after all expenses and taxes have been subtracted from total revenues.
Retained Earnings
Profits that have been reinvested in a company rather than paid out to shareholders as dividends, typically used for business growth or debt payment.
Q5: What is the expected freezing point of
Q18: One mole of which gas has the
Q31: Cesium has a radius of 272 pm
Q75: The heat of vaporization of water at
Q96: The heat of combustion per mole for
Q97: Which equation represents the reaction whose ΔH,represents
Q141: How many grams of KBr are required
Q157: What are the major solute-solvent interactions created
Q159: To make a 2.0 M solution,one could
Q179: Ni has a face-centered unit cell.The number