Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 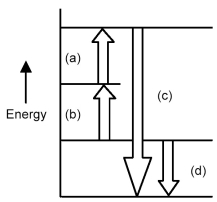
-Which arrow represents ΔHsoln?
Definitions:
Crisis
A critical or difficult situation that requires immediate action for resolution.
Resolution
The act of solving a problem, dispute, or contentious matter, often involving deciding on a course of action or determining a clear outcome.
Bicultural Identity
Identity formation that occurs when adolescents identify in some ways with their ethnic group and in other ways with the majority culture.
Ethnic Culture
The shared practices, values, traditions, and beliefs of a specific ethnic group, distinguishing it from other groups.
Q23: Which of the following solutions will have
Q47: A reaction has the rate law Rate
Q63: Shown below is a concentration vs.time plot
Q87: Which cation in each set is expected
Q124: What is the activation energy for the
Q124: The vapor pressure of liquid chloroform,CHCl<sub>3</sub>,is 400.0
Q140: For the reaction shown below,which change in
Q171: Helium can be liquefied when He atoms
Q175: A saturated solution is defined as<br>A)a concentrated
Q191: For a particular first-order reaction,it takes 24