Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 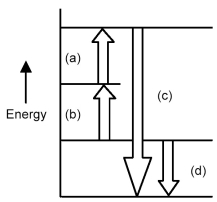
-Which arrows represent ΔHsolute-solute and ΔHsolvent-solvent?
Definitions:
Transtheoretical Model
A theory of behavior change that outlines stages from not considering change to maintaining new behavior, applied in various settings.
Anger Issues
Problems with managing feelings of anger, which can affect an individual's emotional well-being and relationships with others.
Contemplation
A stage in the change process where an individual recognizes a problem and begins to think about resolving it but hasn't committed to action.
Process of Change
The process of change refers to the sequence of stages or actions through which an individual, group, or organization undergoes transformation or adaptation.
Q16: When 15.0 g of zinc metal reacts
Q18: A catalyst increases the rate of a
Q78: Which nonequilibrium mixtures will react in the
Q91: Identify the packing in the figure shown
Q136: A steel bottle contains argon gas at
Q154: Consider a compound that undergoes sublimation at
Q164: What volume of 3.00 M CH<sub>3</sub>OH solution
Q168: Manganese crystallizes in a body-centered cubic structure.What
Q181: Hydrogen gas is collected over water in
Q202: From the following chemical reactions determine the